
论文题目:Self-Optimized Metal-Organic Framework Electrocatalysts with Structural Stability and High Current Tolerance for Water Oxidation
论文作者:Chaopeng Wang, Yang Feng, Hao Sun, Yurou Wang, Jun Yin, Zhenpeng Yao, Xianhe Bu*, Jian Zhu
Abstract:
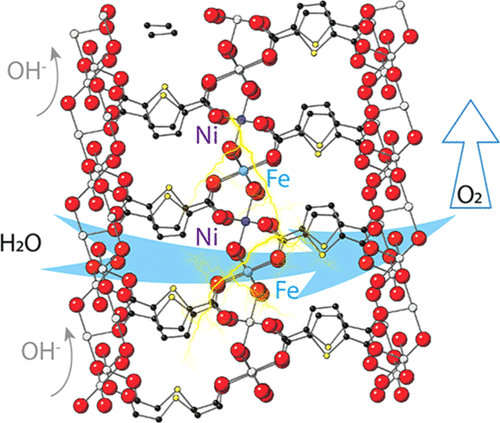
Metal-organic frameworks (MOFs) as electrocatalysts for oxygen evolution reaction (OER) typically suffer from fast degradation under harsh electrolyte conditions, impeding their practical use in industrial electrolyzers. Besides, the evolution of catalytic centers in MOFs and the related influence on their performance along the progress of reaction have rarely been studied. Here, we report a type of structurally stable bimetallic FeNi-MOF nanoarrays with self-optimized electrocatalytic activities in the oxygen production. Such a unique dynamic phenomenon is related with the gradual valence increments of Fe ions in MOFs, which trigger the continuous performance improvement before reaching an optimal steady state. Apart from the intact crystalline structures upon cycling, these FeNi-MOFs achieve low overpotentials of 239 and 308 mV at the current densities of 50 and 200 mA cm(-2), respectively, and show durable operation for over 1033 h (>43 days) at 100 mA cm(-2) and for another 200 h at 500 mA cm(-2). A direct comparison of isostructural and single crystalline Fe-MOFs and Ni-MOFs resolves higher activities of Fe sites in the bimetallic MOFs, which are corroborated by theoretical calculations. The Fe-O bond covalency increment during Fe oxidation enhances the proton-electron transfers with the oxygen 2p-band closer to the Fermi level, thereby expediting the OER process. This work provides deep insights into the understanding of catalytic processes in heterometallic MOFs.
