
论文题目:Confinement in Zeolite and Zeolite Catalysis
论文作者:Yuchao Chai, Weili Dai, Guangjun Wu, Naijia Guan, and Landong Li*
发表期刊:Accounts of Chemical Research , 54(13), 2894-2904, 2021
Abstract:
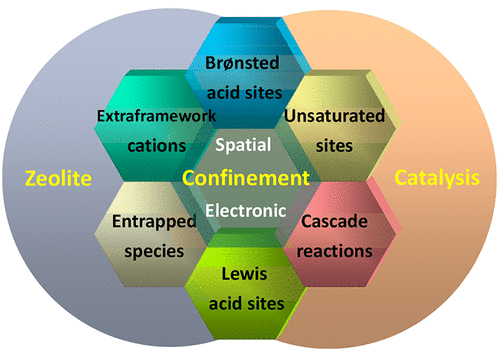
Zeolites, accompanied by their initial discovery as natural mines and the subsequent large-scale commercial production, have played indispensable roles in various fields such as petroleum refining and the chemical industry. Understanding the characteristics of zeolites, in contrast to their counterparts with similar chemical compositions and the origin thereof, is always a hot and challenging topic. Zeolites are known as intrinsic confined systems with ordered channels on the molecular scale, and structural confinement has been proposed to explain the unique chemical behaviors of zeolites. Generally, the channels of zeolites can regulate the diffusion of molecules, leading to a visible difference in molecular transportation and the ultimate shape-selective catalysis. On the other hand, the local electric field within the zeolite channels or cages can act on the guest molecules and change their energy levels. Confinement can be simply interpreted from both spatial and electronic issues; however, the nature of zeolite confinement is ambiguous and needs to be clarified.In this Account, we make a concise summary and analysis of the topics of confinement in a zeolite and zeolite catalysis from two specific views of spatial constraint and a local electric field to answer two basic questions of why zeolites and what else can we do with zeolites. First, it is shown how to construct functional sites including Brønsted acid sites, Lewis acid sites, extraframework cation sites, and entrapped metal or oxide aggregates in zeolites via confinement and how to understand the specific role of confinement in their reactivity. Second, the multiple impacts of confinement in zeolite-catalyzed reactions are discussed, which rationally lead to several unique processes, namely, Brønsted acid catalysis confined in zeolites, Lewis acid catalysis confined in zeolites, catalysis by zeolite-confined coordinatively unsaturated cation sites, and a cascade reaction within the confined space of zeolites. Overall, confinement effects do exist in zeolite systems and have already played extremely important roles in adsorption and catalysis. Although confinement might exist in many systems, the confinement by zeolites is more straightforward thanks to their well-ordered and rigid structure, deriving unique chemical behaviors within the confined space of zeolites. A zeolite is a fantastic scaffold for constructing isolated sites spatially and electrostatically confined in its matrix. Furthermore, zeolites containing well-defined transition-metal sites can be treated as inorganometallic complexes (i.e., a zeolite framework as the ligand of transition-metal ions) and can catalyze reactions resembling organometallic complexes or even metalloenzymes. The local electric field within the confined space of zeolites is strong enough to induce or assist the activation of small molecules, following the working fashion of frustrated Lewis pairs. The tactful utilization of structural confinement, both spatially and electronically, becomes the key to robust zeolites for adsorption and catalysis.
Weili Dai
Weili DaiSchool of Materials Science and Engineering, Nankai University, 38# Tongyan Road, Haihe Education Park, Tianjin 300350, P. R. ChinaMore by Weili Dai, Guangjun Wu
Guangjun WuSchool of Materials Science and Engineering, Nankai University, 38# Tongyan Road, Haihe Education Park, Tianjin 300350, P. R. ChinaMore by Guangjun Wu, Naijia Guan
Naijia GuanSchool of Materials Science and Engineering, Nankai University, 38# Tongyan Road, Haihe Education Park, Tianjin 300350, P. R. ChinaMore by Naijia Guan, and Landong Li*
Landong Li*Email: lild@nankai.edu.cnSchool of Materials Science and Engineering, Nankai University, 38# Tongyan Road, Haihe Education Park, Tianjin 300350, P. R. ChinaFrontiers Science Center for New Organic Matter & Key Laboratory of Advanced Energy Materials Chemistry of Ministry of Education, College of Chemistry, Nankai University, 94# Weijin Road, Nankai District, Tianjin 300071, P. R. ChinaMore by Landong Li

